

About Symphony Trials
Pharmaceutical R&D is long, complex and an expensive process. Symphony Trials can partner with you in the clinical development and testing phase to ensure quality outcomes along with a time-bound management.
At Symphony Trials, we are focused on facilitating and managing the clinical trial efficiently while ensuring the adherence to the desired quality, ethical and regulatory guidelines.
To become your preferred partner for clinical trial and site management services.
To provide best user experience and value through seamless services for the management of clinical trials



Our Range of Services Spans the Following Domains

Symphony Clinical Trial Management Services
We facilitate and manage the clinical trial services for India for local and global clinical trials.
Our expertise, experience and services will combine to address your needs to conduct clinical studies smoothly, efficiently and in a time-bound manner.
Whether you are a large corporate or a CRO, our clinical trial solutions are designed to address your specific needs and deliver a smooth development pathway.
High Performance Clinical Trial Management Services
Our mission is to deliver exceptional clinical trial management services to physicians, clinics, hospitals, and healthcare providers, ensuring accuracy, reliability, and excellence in every test.
Indian clinical trials industry has the required clinical research service capabilities to conduct the clinical trials ethically and efficiently
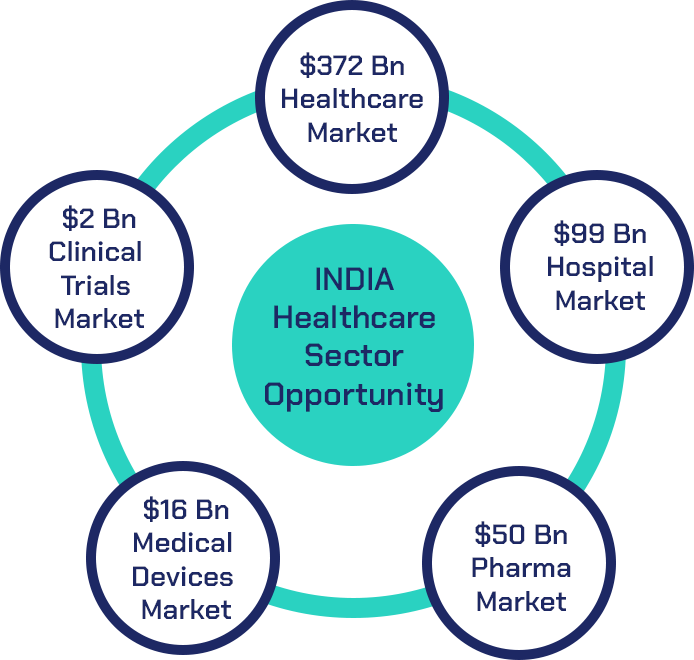
The Indian Healthcare industry achieved a robust growth in 2023 and was valued at USD 372 billion. As of 2024, the Indian healthcare sector is one of India’s largest employers as it employs a total of 7.5 million people.
As per the Economic Survey 2023-24, India’s public expenditure on healthcare touched 1.9 % of GDP in FY24, against 1.6% in FY23,
India’s hospital market was valued at approximately USD 99 billion in 2023 and was estimated to grow at a CAGR of 8.0% from 2024 to 2032.
India’s pharmaceutical market for FY 2023-24 is valued at USD 50 billion with domestic consumption valued at USD 23.5 billion and export valued at USD 26.5 billion. India’s pharma industry is considered to be the world’s third largest by volume and 14th in terms of value of production, producing more than 60,000 generic drugs across 60 therapeutic categories. Indian pharma industry is growing double-digit year on year, and it has achieved the double-digit growth in real terms over the last 10 years.
From generics, India is now moving fast into biosimilars and medtech. Very recently, two Indian companies, Immuneel and ImmunoAct, lauched the CAR-T cell therapies in India.
The Indian clinical trial industry has developed a complete range of clinical research service capabilities that meet global standards, given the vast talent and educated workforce that is available in the country. The available expertise covers medical writing, site management, data management, regulatory submissions and patient recruitment.
Over the past two decades, the clinical trials in India have become easier to conduct given the regulatory transparency, accessibility of sites and availability of medical, clinical and technical workforce.










